- トップ
- > English
- > News
- > 2023
- > Early enhanced skin treatment for eczema resulted in about 25% reduction in hen's egg allergy onset when compared with conventional treatment in infants diagnosed with Atopic dermatitis
Early enhanced skin treatment for eczema resulted in about 25% reduction in hen's egg allergy onset when compared with conventional treatment in infants diagnosed with Atopic dermatitis
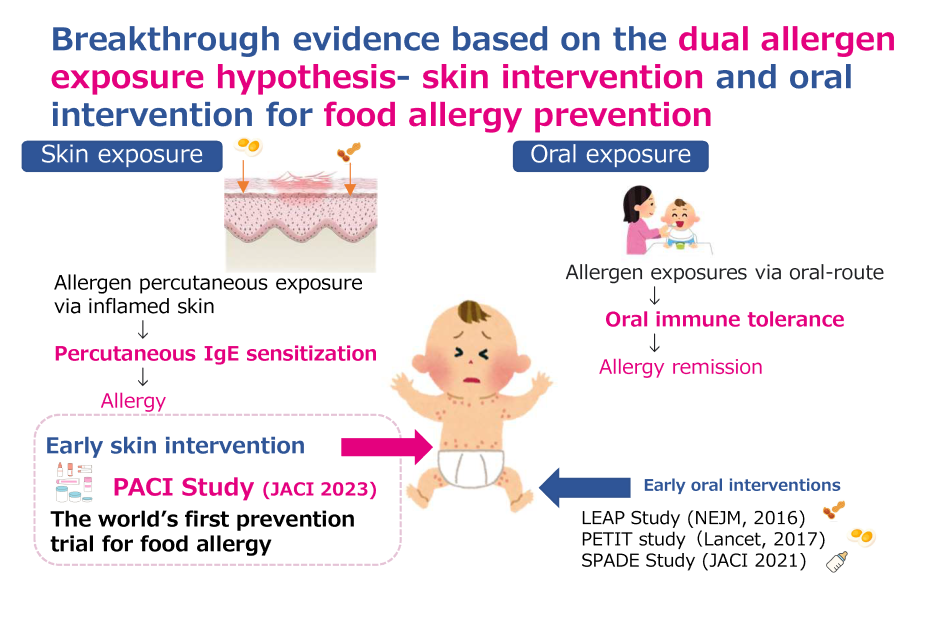
【Fig. 1 Key finding of the PACI Study】
Points of the press release
- We conducted a multicenter, parallel-group, open-label, assessor-blind, randomized controlled trial (PACI Study - Prevention of Allergy via Cutaneous Intervention Study). We enrolled infants aged 7-13 weeks with atopic dermatitis and randomly assigned infants in a 1:1 ratio to enhanced early skin treatment or conventional reactive treatment using topical corticosteroids (TCSs). The primary outcome was the proportion of immediate hen's egg allergy confirmed by oral food challenge at 28 weeks of age.
- Early enhanced treatment applied to both clinically affected and unaffected skin resulted in about 25% reduction in hen's egg allergy onset when compared with conventional treatment only for clinically affected skin in infant atopic dermatitis.
- This study demonstrated for the first time in the world that the onset of food allergy can be prevented by early enhanced skin intervention of atopic dermatitis with the goal of achieving no eczema (EASI100), as suggested by the dual antigen exposure hypothesis.
- However, the early enhanced treatment used in this study should not be applied directly to clinical practice for a prevention strategy. In a real-world setting, a personalized staged approach to atopic dermatitis treatment should be adopted according to the morphology, distribution, severity, and treatment response. The eczema treatment schedule for the potent, duration, and dose of TCSs should be customized for each infant to avoid side effects and achieve no eczema.
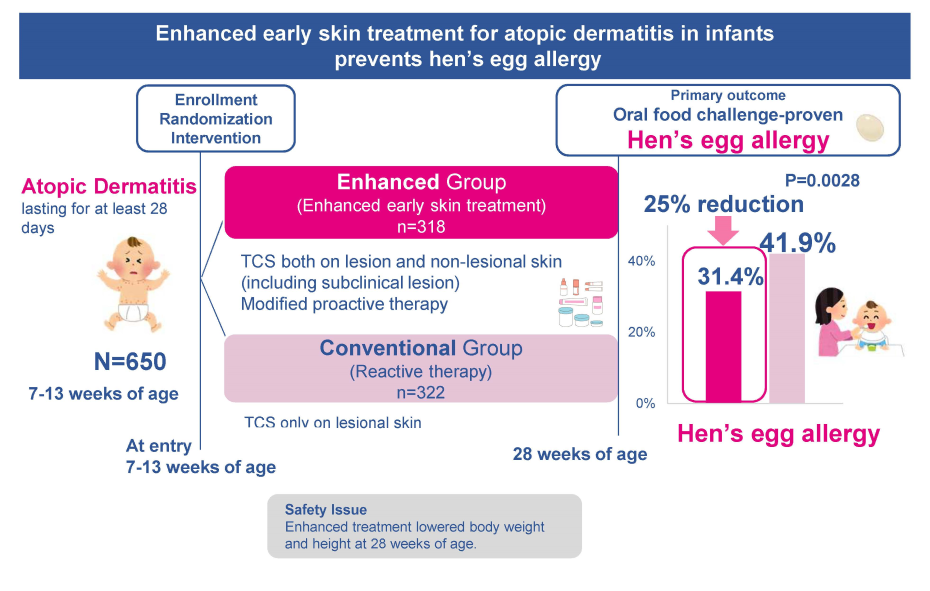
【Fig. 2 Summary of the PACI Study】
Background and Objective
The food allergy epidemic is now a global concern, and its psycho-social and economic burden has become a major public health issue. Peanut is the leading cause of food allergy in North America, whereas hen's egg allergy prevails in Australia and Asian countries. Causal food allergens vary among regions. Atopic dermatitis also confers a heavy burden on quality of life and is a strong risk factor for food allergy.
The dual-allergen-exposure hypothesis implies that early introduction of allergenic foods in infants and early intervention (or prevention) of atopic dermatitis are both important in the prevention of food allergy. The effectiveness of the early introduction of peanut, hen's egg, and multiple allergenic foods has been verified by randomized controlled trials (RCTs) such as the Learning Early about Peanut Allergy (LEAP), the Two-step Egg Introduction for preventing egg allergy in High-risk Infants with eczema (PETIT), the Enquiring About Tolerance (EAT), and the Preventing Atopic Dermatitis and ALLergies in Children (PreventADALL) studies, resulting in the changes in the contents of food allergy management guideline and public implementation.
With respect to early interventions for preventing or treating atopic dermatitis in past several trials, those using only emollients (moisturizers) or pimecrolimus as reactive treatment on affected skin did not prevent food allergy. Emollients and weak anti-inflammatory medication applied only to clinically affected skin lesions might be insufficient to prevent food allergy. Proactive treatment is a long-term maintenance approach using low-dose intermittent anti-inflammatory topical agents such as TCSs to previously affected or usually relapsing body areas. We previously published a retrospective cohort study that early enhanced therapy on both clinical and subclinical skin lesions with the proactive use of betamethasone valerate 0.12% (medium potency in the US standard Pharmacopoeia) reduced the onset of food allergy. However, such an approach has not been confirmed in a large-scale RCT design.
This study aims to ascertain the efficacy of early enhanced proactive treatment applied to both clinically affected and unaffected skin compared with a conventional reactive treatment only for affected skin of infant atopic dermatitis to prevent food allergy.
Study method
This study was a multicenter, parallel-group, open-label, assessor-blind, RCT at 16 hospitals in Japan from July 18, 2017, to February 18, 2021. We enrolled infants aged 7-13 weeks with atopic dermatitis and randomly assigned infants in a 1:1 ratio to enhanced early skin treatment or conventional reactive treatment using TCS. The primary outcome was the presence of immediate hen's egg allergy defined if the results of OFC met the PRACTAL criteria30 within 2 hours after last taking hen's egg powder at the age of 28 weeks (around 5-6 months).
The detailed procedures are described in the published protocol paper (https://ctajournal.biomedcentral.com/articles/10.1186/s13601-018-0233-8), and the full protocol is also available in the Online Repository. Briefly, both groups applied emollients (Heparinoid cream®) twice daily during the whole intervention period. Infants in the enhanced group obtained AD remission induction therapy using TCSs (alclometasone dipropionate 0.1% [low potency in the US standard Pharmacopoeia] for the whole face and betamethasone valerate 0.12% [medium potency in the US standard Pharmacopoeia] for the whole body except scalp and face) twice a day for 14 days from the registration day (Day 0) to Day 14. This was followed in the maintenance period by using the same TCSs two days per week from Day 15 until 28 weeks of age. Infants in the conventional group only applied TCSs reactively to affected skin using alclometasone dipropionate and betamethasone valerate, depending on the severity of the skin rash. If infants in both groups did not achieve remission by applying betamethasone valerate for seven days, they were permitted to use mometasone furoate ointment [high potency in the US standard Pharmacopoeia].
Future outlook/Comments from presenters
Enhanced treatment for infant atopic dermatitis reduced the prevalence of hen's egg allergy suggesting the effectiveness of early and aggressive treatment for earl onset eczema to prevent food allergy. However, this trial protocol might have the expense of reduced body weight and height. In the real-world practice, the selection and application of TCSs should be personalized based on the patient's severity and reaction to the TCSs applied to avoid the adverse effects of TCSs Subsequent observational study named PACI-ON study is underway to explore the long-term effect of the enhanced treatment. The safety and efficacy of using new anti-inflammatory ointments may be tried to prevent food via cutaneous intervention.
Remarks
This study was funded by Japan Agency for Medical Research and Development (Grant Number: JP16ek0410027h0001, JP17ek0410027h0002, JP18ek0410027h0003, JP19ek0410054h001, JP20ek0410054h0002, and JP21ek0410054h0003)
Reference
Authors:Yamamoto-Hanada K, Kobayashi T, Mikami M, Williams HC, Saito H, Saito-Abe M, Sato M, Irahara M, Miyaji Y, Ishikawa F, Tsuchiya K, Tamagawa-Mineoka R, Takaoka Y, Takemura Y, Sato S, Wakiguchi H, Hoshi M, Natsume O, Yamaide F, Seike M, Ohya Y; PACI Study investigators.
Title:Enhanced early skin treatment for atopic dermatitis in infants reduces food allergy
Journal:Journal of Allergy and Clinical Immunology
URL: https://www.sciencedirect.com/science/article/pii/S0091674923003317
About the National Center for Child Health and Development
The National Center for Child Health and Development (NCCHD) was established in 2002 by combining the National Okura Hospital and the National Children's Hospital. The NCCHD's philosophy emphasizes cooperation between its hospital and research wings to promote medical care and research aimed at fostering healthy future generations. The NCCHD is the largest hospital in Japan specializing in pediatrics, perinatal care, obstetrics, and maternal medicine and has 490 beds and an average of about 1000 daily outpatient visits. The NCCHD provides comprehensive and continuous "developmental care" encompassing every stage of life, from the fetus, neonate, infant, toddler, school-aged child, and adolescent to the adult, as well as future generations.
In addition, the center conducts research on elucidating the etiology and pathology of diseases and finding cures while also offering insights on building a society for healthy future generations.
Study sites
Allergy Center, National Center for Child Health and Development(Tokyo, Japan)
Kyoto Prefectural University of Medicine(Kyoto, Japan)
Osaka Habikino Medical Center(Osaka, Japan)
Kindai University Faculty of Medicine Osaka, Japan)
National Hospital Organization Sagamihara National Hospital(Kanagawa, Japan)
Yamaguchi University Graduate School of Medicine(Yamaguchi, Japan)
National Hospital Organization Mie National Hospital(Mie, Japan)
Hamamatsu University School of Medicine (Shizuoka, Japan)
Graduate School of Medicine, Chiba University(Chiba, Japan)
Fujita Health University Bantane Hospital(Aichi, Japan)
Showa General Hospital(Tokyo, Japan)
National Hospital Organization Nagoya Medical Center(Aichi, Japan)
Fujita Health University School of Medicine(Aichi, Japan)
Keio University School of Medicine(Tokyo, Japan)
Tohoku Medical and Pharmaceutical University(Miyagi, Japan)
Saitama City Hospital(Saitama,Japan)
Laboratory center
Tokushima University(Tokushima, Japan)
National Institute for Environmental Studies (Ibaraki, Japan)
Adviser
Hywel Williams, D.Sc., FmedSci
Centre of Evidence-Based Dermatology, School of Medicine, University of Nottingham (U.K.)



